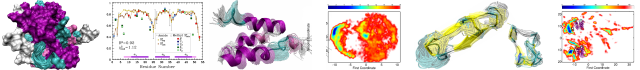Journal of Structural Biology
Austromegabalanus psittacus barnacle shell structure and proteoglycan localization and functionality
Publication date: September 2015
Source:Journal of Structural Biology, Volume 191, Issue 3
Author(s): M.S. Fernández, J.I. Arias, A. Neira-Carrillo, J.L. Arias
Comparative analyzes of biomineralization models have being crucial for the understanding of the functional properties of biominerals and the elucidation of the processes through which biomacromolecules control the synthesis and structural organization of inorganic mineral-based biomaterials. Among calcium carbonate-containing bioceramics, egg, mollusk and echinoderm shells, and crustacean carapaces, have being fairly well characterized. However, Thoraceca barnacles, although being crustacea, showing molting cycle, build a quite stable and heavily mineralized shell that completely surround the animal, which is for life firmly cemented to the substratum. This makes barnacles an interesting model for studying processes of biomineralization. Here we studied the main microstructural and ultrastructural features of Austromegabalanus psittacus barnacle shell, characterize the occurrence of specific proteoglycans (keratan-, dermatan- and chondroitin-6-sulfate proteoglycans) in different soluble and insoluble organic fractions extracted from the shell, and tested them for their ability to crystallize calcium carbonate in vitro. Our results indicate that, in the barnacle model, proteoglycans are good candidates for the modification of the calcite crystal morphology, although the cooperative effect of some additional proteins in the shell could not be excluded.
Source:Journal of Structural Biology, Volume 191, Issue 3
Author(s): M.S. Fernández, J.I. Arias, A. Neira-Carrillo, J.L. Arias
Comparative analyzes of biomineralization models have being crucial for the understanding of the functional properties of biominerals and the elucidation of the processes through which biomacromolecules control the synthesis and structural organization of inorganic mineral-based biomaterials. Among calcium carbonate-containing bioceramics, egg, mollusk and echinoderm shells, and crustacean carapaces, have being fairly well characterized. However, Thoraceca barnacles, although being crustacea, showing molting cycle, build a quite stable and heavily mineralized shell that completely surround the animal, which is for life firmly cemented to the substratum. This makes barnacles an interesting model for studying processes of biomineralization. Here we studied the main microstructural and ultrastructural features of Austromegabalanus psittacus barnacle shell, characterize the occurrence of specific proteoglycans (keratan-, dermatan- and chondroitin-6-sulfate proteoglycans) in different soluble and insoluble organic fractions extracted from the shell, and tested them for their ability to crystallize calcium carbonate in vitro. Our results indicate that, in the barnacle model, proteoglycans are good candidates for the modification of the calcite crystal morphology, although the cooperative effect of some additional proteins in the shell could not be excluded.
Categories: Journal Articles
Exploring the ‘aggregation-prone’ core of human Cystatin C: A structural study
Publication date: September 2015
Source:Journal of Structural Biology, Volume 191, Issue 3
Author(s): Paraskevi L. Tsiolaki, Nikolaos N. Louros, Stavros J. Hamodrakas, Vassiliki A. Iconomidou
Amyloidogenic proteins like human Cystatin C (hCC) have been shown to form dimers and oligomers by exchange of subdomains of the monomeric proteins. Normally, the hCC monomer, a low molecular type 2 Cystatin, consists of 120 amino acid residues and functions as an inhibitor of cysteine proteases. The oligomerization of hCC is involved in the pathophysiology of a rare form of amyloidosis namely Icelandic hereditary cerebral amyloid angiopathy, in which an L68Q mutant is deposited as amyloid in brain arteries of young adults. In order to find the shortest stretch responsible to drive the fibril formation of hCC, we have previously demonstrated that the LQVVR peptide forms amyloid fibrils, in vitro (Tsiolaki et al., 2015). Predictions by AMYLPRED, an amyloidogenic determinant prediction algorithm developed in our lab, led us to synthesize and experimentally study two additional predicted peptides derived from hCC. Along with our previous findings, in this work, we reveal that these peptides self-assemble, in a similar way, into amyloid-like fibrils in vitro, as electron microscopy, X-ray fiber diffraction, ATR FT-IR spectroscopy and Congo red staining studies have shown. Further to our experimental results, all three peptides seem to have a fundamental contribution in forming the “aggregation-prone” core of human Cystatin C.
Source:Journal of Structural Biology, Volume 191, Issue 3
Author(s): Paraskevi L. Tsiolaki, Nikolaos N. Louros, Stavros J. Hamodrakas, Vassiliki A. Iconomidou
Amyloidogenic proteins like human Cystatin C (hCC) have been shown to form dimers and oligomers by exchange of subdomains of the monomeric proteins. Normally, the hCC monomer, a low molecular type 2 Cystatin, consists of 120 amino acid residues and functions as an inhibitor of cysteine proteases. The oligomerization of hCC is involved in the pathophysiology of a rare form of amyloidosis namely Icelandic hereditary cerebral amyloid angiopathy, in which an L68Q mutant is deposited as amyloid in brain arteries of young adults. In order to find the shortest stretch responsible to drive the fibril formation of hCC, we have previously demonstrated that the LQVVR peptide forms amyloid fibrils, in vitro (Tsiolaki et al., 2015). Predictions by AMYLPRED, an amyloidogenic determinant prediction algorithm developed in our lab, led us to synthesize and experimentally study two additional predicted peptides derived from hCC. Along with our previous findings, in this work, we reveal that these peptides self-assemble, in a similar way, into amyloid-like fibrils in vitro, as electron microscopy, X-ray fiber diffraction, ATR FT-IR spectroscopy and Congo red staining studies have shown. Further to our experimental results, all three peptides seem to have a fundamental contribution in forming the “aggregation-prone” core of human Cystatin C.
Categories: Journal Articles
The Ku–Mar zinc finger: A segment-swapped zinc ribbon in MarR-like transcription regulators related to the Ku bridge
Publication date: September 2015
Source:Journal of Structural Biology, Volume 191, Issue 3
Author(s): Gurmeet Kaur, Srikrishna Subramanian
Two putative oxidative-stress sensor proteins from Pseudomonas aeruginosa, PA1607 and PA1374, belong to the MarR family of transcription regulators and possess a unique mode of dimerization. In these proteins, in addition to the α-helices involved in dimerization, inter-subunit contacts are strengthened by additional C-terminal β-strands. Using sequence and structure analysis we show that these β-strands constitute a novel segment-swapped zinc ribbon domain. We detect the presence of the zinc ribbon domain in MarR proteins from many bacterial homologs. While the metal-chelating residues of the zinc ribbons are absent in most members of this family, we could however identify several species of Proteobacteria, Actinobacteria and Firmicutes that possess intact zinc-chelating sites. Conservation pattern of metal-chelating residues together with the extensive structural resemblance to zinc ribbons, in particular to the bridge-region of the dsDNA break repair protein Ku, suggests that the C-terminal β-rich region of these proteins is a zinc ribbon. Sequence analysis also supports a distant evolutionary connection between the zinc ribbons of the MarR and Ku families. However, unlike Ku where the segment-swapped zinc ribbons play a role in DNA-binding and obligate dimerization, their primary role in MarR appears to be in dimerization and strengthening of inter-subunit contacts.
Source:Journal of Structural Biology, Volume 191, Issue 3
Author(s): Gurmeet Kaur, Srikrishna Subramanian
Two putative oxidative-stress sensor proteins from Pseudomonas aeruginosa, PA1607 and PA1374, belong to the MarR family of transcription regulators and possess a unique mode of dimerization. In these proteins, in addition to the α-helices involved in dimerization, inter-subunit contacts are strengthened by additional C-terminal β-strands. Using sequence and structure analysis we show that these β-strands constitute a novel segment-swapped zinc ribbon domain. We detect the presence of the zinc ribbon domain in MarR proteins from many bacterial homologs. While the metal-chelating residues of the zinc ribbons are absent in most members of this family, we could however identify several species of Proteobacteria, Actinobacteria and Firmicutes that possess intact zinc-chelating sites. Conservation pattern of metal-chelating residues together with the extensive structural resemblance to zinc ribbons, in particular to the bridge-region of the dsDNA break repair protein Ku, suggests that the C-terminal β-rich region of these proteins is a zinc ribbon. Sequence analysis also supports a distant evolutionary connection between the zinc ribbons of the MarR and Ku families. However, unlike Ku where the segment-swapped zinc ribbons play a role in DNA-binding and obligate dimerization, their primary role in MarR appears to be in dimerization and strengthening of inter-subunit contacts.
Categories: Journal Articles
The crystal structure of Erwinia amylovora levansucrase provides a snapshot of the products of sucrose hydrolysis trapped into the active site
Publication date: September 2015
Source:Journal of Structural Biology, Volume 191, Issue 3
Author(s): Jochen Wuerges, Lorenzo Caputi, Michele Cianci, Stephane Boivin, Rob Meijers, Stefano Benini
Levansucrases are members of the glycoside hydrolase family and catalyse both the hydrolysis of the substrate sucrose and the transfer of fructosyl units to acceptor molecules. In the presence of sufficient sucrose, this may either lead to the production of fructooligosaccharides or fructose polymers. Aim of this study is to rationalise the differences in the polymerisation properties of bacterial levansucrases and in particular to identify structural features that determine different product spectrum in the levansucrase of the Gram-negative bacterium Erwinia amylovora (Ea Lsc, EC 2.4.1.10) as compared to Gram-positive bacteria such as Bacillus subtilis levansucrase. Ea is an enterobacterial pathogen responsible for the Fire Blight disease in rosaceous plants (e.g., apple and pear) with considerable interest for the agricultural industry. The crystal structure of Ea Lsc was solved at 2.77Å resolution and compared to those of other fructosyltransferases from Gram-positive and Gram-negative bacteria. We propose the structural features, determining the different reaction products, to reside in just a few loops at the rim of the active site funnel. Moreover we propose that loop 8 may have a role in product length determination in Gluconacetobacter diazotrophicus LsdA and Microbacterium saccharophilum FFase. The Ea Lsc structure shows for the first time the products of sucrose hydrolysis still bound in the active site.
Source:Journal of Structural Biology, Volume 191, Issue 3
Author(s): Jochen Wuerges, Lorenzo Caputi, Michele Cianci, Stephane Boivin, Rob Meijers, Stefano Benini
Levansucrases are members of the glycoside hydrolase family and catalyse both the hydrolysis of the substrate sucrose and the transfer of fructosyl units to acceptor molecules. In the presence of sufficient sucrose, this may either lead to the production of fructooligosaccharides or fructose polymers. Aim of this study is to rationalise the differences in the polymerisation properties of bacterial levansucrases and in particular to identify structural features that determine different product spectrum in the levansucrase of the Gram-negative bacterium Erwinia amylovora (Ea Lsc, EC 2.4.1.10) as compared to Gram-positive bacteria such as Bacillus subtilis levansucrase. Ea is an enterobacterial pathogen responsible for the Fire Blight disease in rosaceous plants (e.g., apple and pear) with considerable interest for the agricultural industry. The crystal structure of Ea Lsc was solved at 2.77Å resolution and compared to those of other fructosyltransferases from Gram-positive and Gram-negative bacteria. We propose the structural features, determining the different reaction products, to reside in just a few loops at the rim of the active site funnel. Moreover we propose that loop 8 may have a role in product length determination in Gluconacetobacter diazotrophicus LsdA and Microbacterium saccharophilum FFase. The Ea Lsc structure shows for the first time the products of sucrose hydrolysis still bound in the active site.
Categories: Journal Articles
Effect of fringe-artifact correction on sub-tomogram averaging from Zernike phase-plate cryo-TEM
Publication date: September 2015
Source:Journal of Structural Biology, Volume 191, Issue 3
Author(s): Gregory P. Kishchenko, Radostin Danev, Rebecca Fisher, Jie He, Chyongere Hsieh, Michael Marko, Haixin Sui
Zernike phase-plate (ZPP) imaging greatly increases contrast in cryo-electron microscopy, however fringe artifacts appear in the images. A computational de-fringing method has been proposed, but it has not been widely employed, perhaps because the importance of de-fringing has not been clearly demonstrated. For testing purposes, we employed Zernike phase-plate imaging in a cryo-electron tomographic study of radial-spoke complexes attached to microtubule doublets. We found that the contrast enhancement by ZPP imaging made nonlinear denoising insensitive to the filtering parameters, such that simple low-frequency band-pass filtering made the same improvement in map quality. We employed sub-tomogram averaging, which compensates for the effect of the “missing wedge” and considerably improves map quality. We found that fringes (caused by the abrupt cut-on of the central hole in the phase plate) can lead to incorrect representation of a structure that is well-known from the literature. The expected structure was restored by amplitude scaling, as proposed in the literature. Our results show that de-fringing is an important part of image-processing for cryo-electron tomography of macromolecular complexes with ZPP imaging.
Source:Journal of Structural Biology, Volume 191, Issue 3
Author(s): Gregory P. Kishchenko, Radostin Danev, Rebecca Fisher, Jie He, Chyongere Hsieh, Michael Marko, Haixin Sui
Zernike phase-plate (ZPP) imaging greatly increases contrast in cryo-electron microscopy, however fringe artifacts appear in the images. A computational de-fringing method has been proposed, but it has not been widely employed, perhaps because the importance of de-fringing has not been clearly demonstrated. For testing purposes, we employed Zernike phase-plate imaging in a cryo-electron tomographic study of radial-spoke complexes attached to microtubule doublets. We found that the contrast enhancement by ZPP imaging made nonlinear denoising insensitive to the filtering parameters, such that simple low-frequency band-pass filtering made the same improvement in map quality. We employed sub-tomogram averaging, which compensates for the effect of the “missing wedge” and considerably improves map quality. We found that fringes (caused by the abrupt cut-on of the central hole in the phase plate) can lead to incorrect representation of a structure that is well-known from the literature. The expected structure was restored by amplitude scaling, as proposed in the literature. Our results show that de-fringing is an important part of image-processing for cryo-electron tomography of macromolecular complexes with ZPP imaging.
Categories: Journal Articles
Complementarity and congruence between exact NOEs and traditional NMR probes for spatial decoding of protein dynamics
Publication date: September 2015
Source:Journal of Structural Biology, Volume 191, Issue 3
Author(s): Beat Vögeli, Simon Olsson, Roland Riek, Peter Güntert
The study of the spatial sampling of biomolecules is essential to understanding the structure–dynamics–function relationship. We have established a protocol for the determination of multiple-state ensembles based on exact measurements of the nuclear Overhauser effect (eNOE). The protocol is practical since it does not require any additional data, while all other NMR data sets must be supplemented by NOE restraints. The question arises as to how much structural and dynamics information is shared between the eNOEs and other NMR probes. We compile one of the largest and most diverse NMR data sets of a protein to date consisting of eNOEs, RDCs and J couplings for GB3. We show that the eNOEs improve the back-prediction of RDCs and J couplings, either upon use of more than one state, or in comparison to conventional NOEs. Our findings indicate that the eNOE data is self-consistent, consistent with other data, and that the structural representation with multiple states is warranted.
Source:Journal of Structural Biology, Volume 191, Issue 3
Author(s): Beat Vögeli, Simon Olsson, Roland Riek, Peter Güntert
The study of the spatial sampling of biomolecules is essential to understanding the structure–dynamics–function relationship. We have established a protocol for the determination of multiple-state ensembles based on exact measurements of the nuclear Overhauser effect (eNOE). The protocol is practical since it does not require any additional data, while all other NMR data sets must be supplemented by NOE restraints. The question arises as to how much structural and dynamics information is shared between the eNOEs and other NMR probes. We compile one of the largest and most diverse NMR data sets of a protein to date consisting of eNOEs, RDCs and J couplings for GB3. We show that the eNOEs improve the back-prediction of RDCs and J couplings, either upon use of more than one state, or in comparison to conventional NOEs. Our findings indicate that the eNOE data is self-consistent, consistent with other data, and that the structural representation with multiple states is warranted.
Categories: Journal Articles
A Bayesian approach for suppression of limited angular sampling artifacts in single particle 3D reconstruction
Publication date: September 2015
Source:Journal of Structural Biology, Volume 191, Issue 3
Author(s): Toshio Moriya, Erman Acar, R. Holland Cheng, Ulla Ruotsalainen
In the single particle reconstruction, the initial 3D structure often suffers from the limited angular sampling artifact. Selecting 2D class averages of particle images generally improves the accuracy and efficiency of the reference-free 3D angle estimation, but causes an insufficient angular sampling to fill the information of the target object in the 3D frequency space. Similarly, the initial 3D structure by the random-conical tilt reconstruction has the well-known “missing cone” artifact. Here, we attempted to solve the limited angular sampling problem by sequentially applying maximum a posteriori estimate with expectation maximization algorithm (sMAP-EM). Using both simulated and experimental cryo-electron microscope images, the sMAP-EM was compared to the direct Fourier method on the basis of reconstruction error and resolution. To establish selection criteria of the final regularization weight for the sMAP-EM, the effects of noise level and sampling sparseness on the reconstructions were examined with evenly distributed sampling simulations. The frequency information filled in the missing cone of the conical tilt sampling simulations was assessed by developing new quantitative measurements. All the results of visual and numerical evaluations showed the sMAP-EM performed better than the direct Fourier method, regardless of the sampling method, noise level, and sampling sparseness. Furthermore, the frequency domain analysis demonstrated that the sMAP-EM can fill the meaningful information in the unmeasured angular space without detailed a priori knowledge of the objects. The current research demonstrated that the sMAP-EM has a high potential to facilitate the determination of 3D protein structures at near atomic-resolution.
Source:Journal of Structural Biology, Volume 191, Issue 3
Author(s): Toshio Moriya, Erman Acar, R. Holland Cheng, Ulla Ruotsalainen
In the single particle reconstruction, the initial 3D structure often suffers from the limited angular sampling artifact. Selecting 2D class averages of particle images generally improves the accuracy and efficiency of the reference-free 3D angle estimation, but causes an insufficient angular sampling to fill the information of the target object in the 3D frequency space. Similarly, the initial 3D structure by the random-conical tilt reconstruction has the well-known “missing cone” artifact. Here, we attempted to solve the limited angular sampling problem by sequentially applying maximum a posteriori estimate with expectation maximization algorithm (sMAP-EM). Using both simulated and experimental cryo-electron microscope images, the sMAP-EM was compared to the direct Fourier method on the basis of reconstruction error and resolution. To establish selection criteria of the final regularization weight for the sMAP-EM, the effects of noise level and sampling sparseness on the reconstructions were examined with evenly distributed sampling simulations. The frequency information filled in the missing cone of the conical tilt sampling simulations was assessed by developing new quantitative measurements. All the results of visual and numerical evaluations showed the sMAP-EM performed better than the direct Fourier method, regardless of the sampling method, noise level, and sampling sparseness. Furthermore, the frequency domain analysis demonstrated that the sMAP-EM can fill the meaningful information in the unmeasured angular space without detailed a priori knowledge of the objects. The current research demonstrated that the sMAP-EM has a high potential to facilitate the determination of 3D protein structures at near atomic-resolution.
Categories: Journal Articles
Different binding and recognition modes of GL479, a dual agonist of Peroxisome Proliferator-Activated Receptor α/γ
Publication date: September 2015
Source:Journal of Structural Biology, Volume 191, Issue 3
Author(s): Jademilson Celestino dos Santos, Amanda Bernardes, Letizia Giampietro, Alessandra Ammazzalorso, Barbara De Filippis, Rosa Amoroso, Igor Polikarpov
Peroxisome Proliferator-Activated Receptors (PPARs) are ligand-dependent transcription factors that control various functions in human organism, including the control of glucose and lipid metabolism. PPARγ is a target of TZD agonists, clinically used to improve insulin sensitivity whereas fibrates, PPARα ligands, lower serum triglyceride levels. We report here the structural studies of GL479, a synthetic dual PPARα/γ agonist, designed by a combination of clofibric acid skeleton and a phenyldiazenyl moiety, as bioisosteric replacement of stilbene group, in complex with both PPARα and PPARγ receptors. GL479 was previously reported as a partial agonist of PPARγ and a full agonist of PPARα with high affinity for both PPARs. Our structural studies reveal different binding modes of GL479 to PPARα and PPARγ, which may explain the distinct activation behaviors observed for each receptor. In both cases the ligand interacts with a Tyr located at helix 12 (H12), resulting in the receptor active conformation. In the complex with PPARα, GL479 occupies the same region of the ligand-binding pocket (LBP) observed for other full agonists, whereas GL479 bound to PPARγ displays a new binding mode. Our results indicate a novel region of PPARs LBP that may be explored for the design of partial agonists as well dual PPARα/γ agonists that combine, simultaneously, the therapeutic effects of the treatment of insulin resistance and dyslipidemia.
Source:Journal of Structural Biology, Volume 191, Issue 3
Author(s): Jademilson Celestino dos Santos, Amanda Bernardes, Letizia Giampietro, Alessandra Ammazzalorso, Barbara De Filippis, Rosa Amoroso, Igor Polikarpov
Peroxisome Proliferator-Activated Receptors (PPARs) are ligand-dependent transcription factors that control various functions in human organism, including the control of glucose and lipid metabolism. PPARγ is a target of TZD agonists, clinically used to improve insulin sensitivity whereas fibrates, PPARα ligands, lower serum triglyceride levels. We report here the structural studies of GL479, a synthetic dual PPARα/γ agonist, designed by a combination of clofibric acid skeleton and a phenyldiazenyl moiety, as bioisosteric replacement of stilbene group, in complex with both PPARα and PPARγ receptors. GL479 was previously reported as a partial agonist of PPARγ and a full agonist of PPARα with high affinity for both PPARs. Our structural studies reveal different binding modes of GL479 to PPARα and PPARγ, which may explain the distinct activation behaviors observed for each receptor. In both cases the ligand interacts with a Tyr located at helix 12 (H12), resulting in the receptor active conformation. In the complex with PPARα, GL479 occupies the same region of the ligand-binding pocket (LBP) observed for other full agonists, whereas GL479 bound to PPARγ displays a new binding mode. Our results indicate a novel region of PPARs LBP that may be explored for the design of partial agonists as well dual PPARα/γ agonists that combine, simultaneously, the therapeutic effects of the treatment of insulin resistance and dyslipidemia.
Categories: Journal Articles
The proteomics of wool fibre morphogenesis
Publication date: September 2015
Source:Journal of Structural Biology, Volume 191, Issue 3
Author(s): Jeffrey E. Plowman, Duane P. Harland, Sivasangary Ganeshan, Joy L. Woods, Bede van Shaijik, Santanu Deb-Choudhury, Ancy Thomas, Stefan Clerens, David R. Scobie
Gel and gel-free proteomic techniques have been used for the first time to directly study the proteins present in whole wool follicles and dissected portions of follicles that correlated with morphological changes in the developing fibre as determined by transmission electron microscopy. Individual wool follicles were dissected into four portions designated as the bulb, elongation, keratogenous and keratinisation portions. Gel-free proteomic analysis of dissected portions from 30 follicles showed that the first keratins to appear were K31, K35 and K85, in the bulb portion. The first epithelial KAP, trichohyalin, was detected in the bulb portion and the first cortical KAP, KAP11.1 was found in the elongation portion. Other major trichocyte keratins and cortical KAPs began to appear further up the follicle in the keratogenous and keratinisation zones. These results were consistent with what has been observed from gene expression studies and correlated well with the morphological changes observed in the follicle. Other proteins detected by this approach included the keratin anchor protein desmoplakin, as well as vimentin and epithelial keratins, histones, ribosomal proteins and collagens. Two-dimensional electrophoretic (2DE) analysis of dissected portions of 50 follicles revealed substantial changes in the position, number and intensity of the spots of the trichocyte keratins as they progressed through the follicle zones, suggesting that they are subject to modification as a result of the keratinisation process. Also present in the 2DE maps were a number of epithelial keratins, presumably from the inner and outer root sheaths, and the dermal components.
Source:Journal of Structural Biology, Volume 191, Issue 3
Author(s): Jeffrey E. Plowman, Duane P. Harland, Sivasangary Ganeshan, Joy L. Woods, Bede van Shaijik, Santanu Deb-Choudhury, Ancy Thomas, Stefan Clerens, David R. Scobie
Gel and gel-free proteomic techniques have been used for the first time to directly study the proteins present in whole wool follicles and dissected portions of follicles that correlated with morphological changes in the developing fibre as determined by transmission electron microscopy. Individual wool follicles were dissected into four portions designated as the bulb, elongation, keratogenous and keratinisation portions. Gel-free proteomic analysis of dissected portions from 30 follicles showed that the first keratins to appear were K31, K35 and K85, in the bulb portion. The first epithelial KAP, trichohyalin, was detected in the bulb portion and the first cortical KAP, KAP11.1 was found in the elongation portion. Other major trichocyte keratins and cortical KAPs began to appear further up the follicle in the keratogenous and keratinisation zones. These results were consistent with what has been observed from gene expression studies and correlated well with the morphological changes observed in the follicle. Other proteins detected by this approach included the keratin anchor protein desmoplakin, as well as vimentin and epithelial keratins, histones, ribosomal proteins and collagens. Two-dimensional electrophoretic (2DE) analysis of dissected portions of 50 follicles revealed substantial changes in the position, number and intensity of the spots of the trichocyte keratins as they progressed through the follicle zones, suggesting that they are subject to modification as a result of the keratinisation process. Also present in the 2DE maps were a number of epithelial keratins, presumably from the inner and outer root sheaths, and the dermal components.
Categories: Journal Articles
Short-time dynamics of pH-dependent conformation and substrate binding in the active site of beta-glucosidases: A computational study
Publication date: September 2015
Source:Journal of Structural Biology, Volume 191, Issue 3
Author(s): David F. Flannelly, Thalia G. Aoki, Ludmilla Aristilde
The complete degradation of cellulose to glucose is essential to carbon turnover in terrestrial ecosystems and to engineered biofuel production. A rate-limiting step in this pathway is catalyzed by beta-glucosidase (BG) enzymes, which convert cellulobiose into two glucose molecules. The activity of these enzymes has been shown to vary with solution pH. However, it is not well understood how pH influences the enzyme conformation required for catalytic action on the substrate. A structural understanding of this pH effect is important for predicting shifts in BG activity in bioreactors and environmental matrices, in addition to informing targeted protein engineering. Here we applied molecular dynamics simulations to explore conformational and substrate binding dynamics in two well-characterized BGs of bacterial (Clostridium cellulovorans) and fungal (Trichoderma reesei) origins as a function of pH. The enzymes were simulated in an explicit solvated environment, with NaCl as electrolytes, at their prominent ionization states obtained at pH 5, 6, 7, and 7.5. Our findings indicated that pH-dependent changes in the ionization states of non-catalytic residues localized outside of the immediate active site led to pH-dependent disruption of the active site conformation. This disruption interferes with favorable H-bonding interactions with catalytic residues required to initiate catalysis on the substrate. We also identified specific non-catalytic residues that are involved in stabilizing the substrate at the optimal pH for enzyme activity. The simulations further revealed the dynamics of water-bridging interactions both outside and inside the substrate binding cleft during structural changes in the enzyme-substrate complex. These findings provide new structural insights into the pH-dependent substrate binding specificity in BGs.
Source:Journal of Structural Biology, Volume 191, Issue 3
Author(s): David F. Flannelly, Thalia G. Aoki, Ludmilla Aristilde
The complete degradation of cellulose to glucose is essential to carbon turnover in terrestrial ecosystems and to engineered biofuel production. A rate-limiting step in this pathway is catalyzed by beta-glucosidase (BG) enzymes, which convert cellulobiose into two glucose molecules. The activity of these enzymes has been shown to vary with solution pH. However, it is not well understood how pH influences the enzyme conformation required for catalytic action on the substrate. A structural understanding of this pH effect is important for predicting shifts in BG activity in bioreactors and environmental matrices, in addition to informing targeted protein engineering. Here we applied molecular dynamics simulations to explore conformational and substrate binding dynamics in two well-characterized BGs of bacterial (Clostridium cellulovorans) and fungal (Trichoderma reesei) origins as a function of pH. The enzymes were simulated in an explicit solvated environment, with NaCl as electrolytes, at their prominent ionization states obtained at pH 5, 6, 7, and 7.5. Our findings indicated that pH-dependent changes in the ionization states of non-catalytic residues localized outside of the immediate active site led to pH-dependent disruption of the active site conformation. This disruption interferes with favorable H-bonding interactions with catalytic residues required to initiate catalysis on the substrate. We also identified specific non-catalytic residues that are involved in stabilizing the substrate at the optimal pH for enzyme activity. The simulations further revealed the dynamics of water-bridging interactions both outside and inside the substrate binding cleft during structural changes in the enzyme-substrate complex. These findings provide new structural insights into the pH-dependent substrate binding specificity in BGs.
Categories: Journal Articles
A New Protocol to Accurately Determine Microtubule Lattice Seam Location
Publication date: Available online 28 September 2015
Source:Journal of Structural Biology
Author(s): Rui Zhang, Eva Nogales
Microtubules (MTs) are cylindrical polymers of αβ-tubulin that display pseudo-helical symmetry due to the presence of a lattice seam of heterologous lateral contacts. The structural similarity between α- and β-tubulin makes it difficult to computationally distinguish them in the noisy cryo-EM images, unless a marker protein for the tubulin dimer, such as kinesin motor domain, is present. We have developed a new data processing protocol that can accurately determine αβ-tubulin register and seam location for MT segments. Our strategy can deal with difficult situations, where the marker protein is relatively small or the decoration of marker protein is sparse. Using this new seam-search protocol, combined with movie processing for data from a direct electron detection camera, we were able to determine the cryo-EM structures of MT at 3.5 Å resolution in different functional states. The successful distinction of α- and β-tubulin allowed us to visualize the nucleotide state at the E-site and the configuration of lateral contacts at the seam.
Source:Journal of Structural Biology
Author(s): Rui Zhang, Eva Nogales
Microtubules (MTs) are cylindrical polymers of αβ-tubulin that display pseudo-helical symmetry due to the presence of a lattice seam of heterologous lateral contacts. The structural similarity between α- and β-tubulin makes it difficult to computationally distinguish them in the noisy cryo-EM images, unless a marker protein for the tubulin dimer, such as kinesin motor domain, is present. We have developed a new data processing protocol that can accurately determine αβ-tubulin register and seam location for MT segments. Our strategy can deal with difficult situations, where the marker protein is relatively small or the decoration of marker protein is sparse. Using this new seam-search protocol, combined with movie processing for data from a direct electron detection camera, we were able to determine the cryo-EM structures of MT at 3.5 Å resolution in different functional states. The successful distinction of α- and β-tubulin allowed us to visualize the nucleotide state at the E-site and the configuration of lateral contacts at the seam.
Categories: Journal Articles
Structure of Liver Receptor Homolog-1 (NR5A2) with PIP3 hormone bound in the ligand binding pocket
Publication date: Available online 28 September 2015
Source:Journal of Structural Biology
Author(s): Elena P. Sablin, Raymond D. Blind, Rubatharshini Uthayaruban, H.J. Chiu, Ashley M. Deacon, Debanu Das, Holly A. Ingraham, Robert J. Fletterick
The nuclear receptor LRH-1 (Liver Receptor Homolog-1, NR5A2) is a transcription factor that regulates gene expression programs critical for many aspects of metabolism and reproduction. Although LRH-1 is able to bind phospholipids, it is still considered an orphan nuclear receptor (NR) with an unknown regulatory hormone. Our prior cellular and structural studies demonstrated that the signaling phosphatidylinositols PI(4,5)P2 (PIP2) and PI(3,4,5)P3 (PIP3) bind and regulate SF-1 (Steroidogenic Factor-1, NR5A1), a close homolog of LRH-1. Here, we describe the crystal structure of human LRH-1 ligand binding domain (LBD) bound by PIP3 - the first phospholipid with a head group endogenous to mammals. We show that the phospholipid hormone binds LRH-1 with high affinity, stabilizing the receptor LBD. While the hydrophobic PIP3 tails (C16/C16) are buried inside the LRH-1 ligand binding pocket, the negatively charged PIP3 head group is presented on the receptor surface, similar to the phosphatidylinositol binding mode observed in the PIP3-SF-1 structure. Thus, data presented in this work reinforce our earlier findings demonstrating that signaling phosphatidylinositols regulate the NR5A receptors LRH-1 and SF-1.
Source:Journal of Structural Biology
Author(s): Elena P. Sablin, Raymond D. Blind, Rubatharshini Uthayaruban, H.J. Chiu, Ashley M. Deacon, Debanu Das, Holly A. Ingraham, Robert J. Fletterick
The nuclear receptor LRH-1 (Liver Receptor Homolog-1, NR5A2) is a transcription factor that regulates gene expression programs critical for many aspects of metabolism and reproduction. Although LRH-1 is able to bind phospholipids, it is still considered an orphan nuclear receptor (NR) with an unknown regulatory hormone. Our prior cellular and structural studies demonstrated that the signaling phosphatidylinositols PI(4,5)P2 (PIP2) and PI(3,4,5)P3 (PIP3) bind and regulate SF-1 (Steroidogenic Factor-1, NR5A1), a close homolog of LRH-1. Here, we describe the crystal structure of human LRH-1 ligand binding domain (LBD) bound by PIP3 - the first phospholipid with a head group endogenous to mammals. We show that the phospholipid hormone binds LRH-1 with high affinity, stabilizing the receptor LBD. While the hydrophobic PIP3 tails (C16/C16) are buried inside the LRH-1 ligand binding pocket, the negatively charged PIP3 head group is presented on the receptor surface, similar to the phosphatidylinositol binding mode observed in the PIP3-SF-1 structure. Thus, data presented in this work reinforce our earlier findings demonstrating that signaling phosphatidylinositols regulate the NR5A receptors LRH-1 and SF-1.
Categories: Journal Articles
Crystal structure of halogenase PltA from the pyoluteorin biosynthetic pathway
Publication date: Available online 28 September 2015
Source:Journal of Structural Biology
Author(s): Allan H. Pang, Sylvie Garneau-Tsodikova, Oleg V. Tsodikov
Pyoluteorin is an antifungal agent composed of a 4,5-dichlorinated pyrrole group linked to a resorcinol moiety. The pyoluteorin biosynthetic gene cluster in Pseudomonas fluorescens Pf-5 encodes the halogenase PltA, which has been previously demonstrated to perform both chlorinations in vitro. PltA selectively accepts as a substrate a pyrrole moiety covalently tethered to a nonribosomal peptide thiolation domain PltL (pyrrolyl-S-PltL) for FAD-dependent di-chlorination, yielding 4,5-dichloropyrrolyl-S-PltL. We report a 2.75 Å-resolution crystal structure of PltA in complex with FAD and chloride. PltA is a dimeric enzyme, containing a flavin-binding fold conserved in flavin-dependent halogenases and monooxygenases, and an additional unique helical region at the C-terminus. This C-terminal region blocks a putative substrate-binding cleft, suggesting that a conformational change involving repositioning of this region is necessary to allow binding of the pyrrolyl-S-PltL substrate for its dichlorination by PltA.
Source:Journal of Structural Biology
Author(s): Allan H. Pang, Sylvie Garneau-Tsodikova, Oleg V. Tsodikov
Pyoluteorin is an antifungal agent composed of a 4,5-dichlorinated pyrrole group linked to a resorcinol moiety. The pyoluteorin biosynthetic gene cluster in Pseudomonas fluorescens Pf-5 encodes the halogenase PltA, which has been previously demonstrated to perform both chlorinations in vitro. PltA selectively accepts as a substrate a pyrrole moiety covalently tethered to a nonribosomal peptide thiolation domain PltL (pyrrolyl-S-PltL) for FAD-dependent di-chlorination, yielding 4,5-dichloropyrrolyl-S-PltL. We report a 2.75 Å-resolution crystal structure of PltA in complex with FAD and chloride. PltA is a dimeric enzyme, containing a flavin-binding fold conserved in flavin-dependent halogenases and monooxygenases, and an additional unique helical region at the C-terminus. This C-terminal region blocks a putative substrate-binding cleft, suggesting that a conformational change involving repositioning of this region is necessary to allow binding of the pyrrolyl-S-PltL substrate for its dichlorination by PltA.
Categories: Journal Articles
Numerical Geometry of Map and Model Assessment
Publication date: Available online 28 September 2015
Source:Journal of Structural Biology
Author(s): Willy Wriggers, Jing He
We are describing best practices and assessment strategies for the atomic interpretation of cryo-Electron Microscopy (cryo-EM) maps. Multiscale numerical geometry strategies in the Situs package and in secondary structure detection software are currently evolving due to the recent increases in cryo-EM resolution. Criteria that aim to predict the accuracy of fitted atomic models at low (worse than 8 Å) and medium (4-8 Å) resolution remain challenging. However, a high level of confidence in atomic models can be achieved by combining such criteria. The observed errors are due to map-model discrepancies and due to the effect of imperfect global docking strategies. Extending the earlier motion capture approach developed for flexible fitting, we use simulated fiducials (pseudoatoms) at varying levels of coarse graining to track the local drift of structural features. We compare three tracking approaches: naïve vector quantization, a smoothly deformable model, and a tessellation of the structure into rigid Voronoi cells which are fitted using a multi-fragment refinement approach. The lowest error is an upper bound for the (small) discrepancy between crystal structure and EM map due to different conditions in their structure determination. When internal features such as secondary structures are visible in medium-resolution EM maps, it is possible to extend the idea of point-based fiducials to more complex geometric representations such as helical axes, strands, and skeletons. We propose a quantitative strategies to assess map-model pairs when such secondary structure patterns are prominent.
Source:Journal of Structural Biology
Author(s): Willy Wriggers, Jing He
We are describing best practices and assessment strategies for the atomic interpretation of cryo-Electron Microscopy (cryo-EM) maps. Multiscale numerical geometry strategies in the Situs package and in secondary structure detection software are currently evolving due to the recent increases in cryo-EM resolution. Criteria that aim to predict the accuracy of fitted atomic models at low (worse than 8 Å) and medium (4-8 Å) resolution remain challenging. However, a high level of confidence in atomic models can be achieved by combining such criteria. The observed errors are due to map-model discrepancies and due to the effect of imperfect global docking strategies. Extending the earlier motion capture approach developed for flexible fitting, we use simulated fiducials (pseudoatoms) at varying levels of coarse graining to track the local drift of structural features. We compare three tracking approaches: naïve vector quantization, a smoothly deformable model, and a tessellation of the structure into rigid Voronoi cells which are fitted using a multi-fragment refinement approach. The lowest error is an upper bound for the (small) discrepancy between crystal structure and EM map due to different conditions in their structure determination. When internal features such as secondary structures are visible in medium-resolution EM maps, it is possible to extend the idea of point-based fiducials to more complex geometric representations such as helical axes, strands, and skeletons. We propose a quantitative strategies to assess map-model pairs when such secondary structure patterns are prominent.
Categories: Journal Articles
The role of the C-terminus and Kpn loop in the quaternary structure stability of nucleoside diphosphate kinase from Leishmania parasites
Publication date: Available online 26 September 2015
Source:Journal of Structural Biology
Author(s): Plínio Salmazo Vieira, Priscila Oliveira de Giuseppe, Arthur Henrique Cavalcante de Oliveira, Mario Tyago Murakami
Nucleoside diphosphate kinase (NDK) is a housekeeping enzyme that plays key roles in nucleotide recycling and homeostasis in trypanosomatids. Moreover, it is secreted by the intracellular parasite Leishmania to modulate the host response. These functions make NDK an attractive target for drug design and for studies aiming at a better understanding of the mechanisms mediating host-pathogen interactions. Here, we report the crystal structures of three mutants of the NDK from Leishmania major (LmNDK) that affects the stability of the hexameric biological assembly including P95S, Δ5Ct (lacking the last five residues) and the double mutant P100S/Δ5Ct. Although P95S and Δ5Ct variants conserve the hexameric structure of the wild-type protein, the double mutant becomes a dimer as shown by in solution studies. Free energy calculation of dimer-dimer interfaces and enzymatic assays indicate that P95S, Δ5Ct and P100S/Δ5Ct mutations progressively decrease the hexamer stability and enzyme activity. These results demonstrate that the mutated regions play a role in protein function through stabilizing the quaternary arrangement.
Source:Journal of Structural Biology
Author(s): Plínio Salmazo Vieira, Priscila Oliveira de Giuseppe, Arthur Henrique Cavalcante de Oliveira, Mario Tyago Murakami
Nucleoside diphosphate kinase (NDK) is a housekeeping enzyme that plays key roles in nucleotide recycling and homeostasis in trypanosomatids. Moreover, it is secreted by the intracellular parasite Leishmania to modulate the host response. These functions make NDK an attractive target for drug design and for studies aiming at a better understanding of the mechanisms mediating host-pathogen interactions. Here, we report the crystal structures of three mutants of the NDK from Leishmania major (LmNDK) that affects the stability of the hexameric biological assembly including P95S, Δ5Ct (lacking the last five residues) and the double mutant P100S/Δ5Ct. Although P95S and Δ5Ct variants conserve the hexameric structure of the wild-type protein, the double mutant becomes a dimer as shown by in solution studies. Free energy calculation of dimer-dimer interfaces and enzymatic assays indicate that P95S, Δ5Ct and P100S/Δ5Ct mutations progressively decrease the hexamer stability and enzyme activity. These results demonstrate that the mutated regions play a role in protein function through stabilizing the quaternary arrangement.
Categories: Journal Articles
Structural and functional analysis of BB0689 from Borrelia burgdorferi, a member of the bacterial CAP superfamily
Publication date: Available online 25 September 2015
Source:Journal of Structural Biology
Author(s): Kalvis Brangulis, Kristaps Jaudzems, Ivars Petrovskis, Inara Akopjana, Andris Kazaks, Kaspars Tars
Spirochete Borrelia burgdorferi is the causative agent of Lyme disease and is transmitted from infected Ixodes ticks to a mammalian host after a tick bite. The outer surface protein BB0689 from B. burgdorferi is up-regulated when the tick feeds, which indicates a potential role for BB0689 in Lyme disease pathogenesis. We have determined the crystal structure of BB0689, which revealed that the protein belongs to the CAP superfamily. Though the CAP domain is widespread in all three cellular domains of life, thus far the CAP domain has been studied only in eukaryotes, in which it is usually linked to certain other domains to form a multi-domain protein and is associated with the mammalian reproductive tract, the plant response to pathogens, venom allergens from insects and reptiles, and the growth of human brain tumors. Though the exact function of the isolated CAP domain remains ambiguous, several functions, including the binding of cholesterol, lipids and heparan sulfate, have been recently attributed to different CAP domain proteins. In this study, the bacterial CAP domain structure was analyzed and compared with the previously solved crystal structures of representative CAPs, and the function of BB0689 was examined. To determine the potential function of BB0689 and ascertain whether the functions that have been attributed to the CAP domain proteins are conserved, the binding of previously reported CAP domain interaction partners was analyzed, and the results suggested that BB0689 has a unique function that is yet to be discovered.
Source:Journal of Structural Biology
Author(s): Kalvis Brangulis, Kristaps Jaudzems, Ivars Petrovskis, Inara Akopjana, Andris Kazaks, Kaspars Tars
Spirochete Borrelia burgdorferi is the causative agent of Lyme disease and is transmitted from infected Ixodes ticks to a mammalian host after a tick bite. The outer surface protein BB0689 from B. burgdorferi is up-regulated when the tick feeds, which indicates a potential role for BB0689 in Lyme disease pathogenesis. We have determined the crystal structure of BB0689, which revealed that the protein belongs to the CAP superfamily. Though the CAP domain is widespread in all three cellular domains of life, thus far the CAP domain has been studied only in eukaryotes, in which it is usually linked to certain other domains to form a multi-domain protein and is associated with the mammalian reproductive tract, the plant response to pathogens, venom allergens from insects and reptiles, and the growth of human brain tumors. Though the exact function of the isolated CAP domain remains ambiguous, several functions, including the binding of cholesterol, lipids and heparan sulfate, have been recently attributed to different CAP domain proteins. In this study, the bacterial CAP domain structure was analyzed and compared with the previously solved crystal structures of representative CAPs, and the function of BB0689 was examined. To determine the potential function of BB0689 and ascertain whether the functions that have been attributed to the CAP domain proteins are conserved, the binding of previously reported CAP domain interaction partners was analyzed, and the results suggested that BB0689 has a unique function that is yet to be discovered.
Categories: Journal Articles
X-Ray recordings reveal how a human disease-linked skeletal muscle α-actin mutation leads to contractile dysfunction
Publication date: Available online 25 September 2015
Source:Journal of Structural Biology
Author(s): Julien Ochala, Gianina Ravenscroft, Elyshia McNamara, Kristen J. Nowak, Hiroyuki Iwamoto
In humans, mutant skeletal muscle α-actin proteins are associated with contractile dysfunction, skeletal muscle weakness and a wide range of primarily skeletal muscle diseases. Despite this knowledge, the exact molecular mechanisms triggering the contractile dysfunction remain unknown. Here, we aimed to unravel these. Hence, we used a transgenic mouse model expressing a well-described D286G mutant skeletal muscle α-actin protein and recapitulating the human condition of contractile deregulation and severe skeletal muscle weakness. We then recorded and analysed the small-angle x-ray diffraction patterns of isolated membrane-permeabilized myofibres. Results showed that upon addition of Ca2+, the intensity changes of the second (1/19 nm−1) and sixth (1/5.9 nm−1) actin layer lines and of the first myosin meridional reflection (1/14.3 nm-1) were disrupted when the thin-thick filament overlap was optimal (sarcomere length of 2.5-2.6 μm). However these reflections were normal when the thin and thick filaments were not interacting (sarcomere length >3.6 μm). These findings demonstrate, for the first time, that the replacement of just one amino acid in the skeletal muscle α-actin protein partly prevents actin conformational changes during activation, disrupting the strong binding of myosin molecules. This leads to a limited myosin-related tropomyosin movement over the thin filaments, further affecting the amount of cross-bridges, explaining the contractile dysfunction.
Graphical abstract
Source:Journal of Structural Biology
Author(s): Julien Ochala, Gianina Ravenscroft, Elyshia McNamara, Kristen J. Nowak, Hiroyuki Iwamoto
In humans, mutant skeletal muscle α-actin proteins are associated with contractile dysfunction, skeletal muscle weakness and a wide range of primarily skeletal muscle diseases. Despite this knowledge, the exact molecular mechanisms triggering the contractile dysfunction remain unknown. Here, we aimed to unravel these. Hence, we used a transgenic mouse model expressing a well-described D286G mutant skeletal muscle α-actin protein and recapitulating the human condition of contractile deregulation and severe skeletal muscle weakness. We then recorded and analysed the small-angle x-ray diffraction patterns of isolated membrane-permeabilized myofibres. Results showed that upon addition of Ca2+, the intensity changes of the second (1/19 nm−1) and sixth (1/5.9 nm−1) actin layer lines and of the first myosin meridional reflection (1/14.3 nm-1) were disrupted when the thin-thick filament overlap was optimal (sarcomere length of 2.5-2.6 μm). However these reflections were normal when the thin and thick filaments were not interacting (sarcomere length >3.6 μm). These findings demonstrate, for the first time, that the replacement of just one amino acid in the skeletal muscle α-actin protein partly prevents actin conformational changes during activation, disrupting the strong binding of myosin molecules. This leads to a limited myosin-related tropomyosin movement over the thin filaments, further affecting the amount of cross-bridges, explaining the contractile dysfunction.
Graphical abstract
Categories: Journal Articles
Site-specific labeling of proteins for electron microscopy
Publication date: Available online 25 September 2015
Source:Journal of Structural Biology
Author(s): Corey M. Dambacher, Gabriel C. Lander
Electron microscopy is commonly employed to determine the subunit organization of large macromolecular assemblies. However, the field lacks a robust molecular labeling methodology for unambiguous identification of constituent subunits. We present a strategy that exploits the unique properties of an unnatural amino acid in order to enable site-specific attachment of a single, readily identifiable protein label at any solvent-exposed position on the macromolecular surface. Using this method, we show clear labeling of a subunit within the 19S proteasome lid subcomplex that has not been amenable to labeling by traditional approaches.
Source:Journal of Structural Biology
Author(s): Corey M. Dambacher, Gabriel C. Lander
Electron microscopy is commonly employed to determine the subunit organization of large macromolecular assemblies. However, the field lacks a robust molecular labeling methodology for unambiguous identification of constituent subunits. We present a strategy that exploits the unique properties of an unnatural amino acid in order to enable site-specific attachment of a single, readily identifiable protein label at any solvent-exposed position on the macromolecular surface. Using this method, we show clear labeling of a subunit within the 19S proteasome lid subcomplex that has not been amenable to labeling by traditional approaches.
Categories: Journal Articles
The influence of frame alignment with dose compensation on the quality of single particle reconstructions
Publication date: Available online 25 September 2015
Source:Journal of Structural Biology
Author(s): John M. Spear, Alex J. Noble, Qing Xie, Duncan R. Sousa, Michael S. Chapman, Scott M. Stagg
As direct electron detection devices in cryo-electron microscopy become ubiquitous, the field is now ripe for new developments in image analysis techniques that take advantage of their increased SNR coupled with their high-throughput frame collection abilities. In approaching atomic resolution of native-like biomolecules, the accurate extraction of structural locations and orientations of side-chains from frames depends not only on the electron dose that a sample receives but also on the ability to accurately estimate the CTF. Here we use a new 2.8Å resolution structure of a recombinant gene therapy virus, AAV-DJ with Arixtra, imaged on an FEI Titan Krios with a DE-20 direct electron detector to probe new metrics including relative side-chain density and ResLog analysis for optimizing the compensation of electron beam damage and to characterize the factors that are limiting the resolution of the reconstruction. The influence of dose compensation on the accuracy of CTF estimation and particle classifiability are also presented. We show that rigorous dose compensation allows for better particle classifiability and greater recovery of structural information from negatively charged, electron-sensitive side-chains, resulting in a more accurate macromolecular model.
Source:Journal of Structural Biology
Author(s): John M. Spear, Alex J. Noble, Qing Xie, Duncan R. Sousa, Michael S. Chapman, Scott M. Stagg
As direct electron detection devices in cryo-electron microscopy become ubiquitous, the field is now ripe for new developments in image analysis techniques that take advantage of their increased SNR coupled with their high-throughput frame collection abilities. In approaching atomic resolution of native-like biomolecules, the accurate extraction of structural locations and orientations of side-chains from frames depends not only on the electron dose that a sample receives but also on the ability to accurately estimate the CTF. Here we use a new 2.8Å resolution structure of a recombinant gene therapy virus, AAV-DJ with Arixtra, imaged on an FEI Titan Krios with a DE-20 direct electron detector to probe new metrics including relative side-chain density and ResLog analysis for optimizing the compensation of electron beam damage and to characterize the factors that are limiting the resolution of the reconstruction. The influence of dose compensation on the accuracy of CTF estimation and particle classifiability are also presented. We show that rigorous dose compensation allows for better particle classifiability and greater recovery of structural information from negatively charged, electron-sensitive side-chains, resulting in a more accurate macromolecular model.
Categories: Journal Articles
Structural analysis of human RPC32β - RPC62 complex
Publication date: Available online 21 September 2015
Source:Journal of Structural Biology
Author(s): Fanny Boissier, Hélène Dumay-Odelot, Martin Teichmann, Sébastien Fribourg
Transcription initiation by eukaryotic RNA polymerase (Pol) III relies on the subcomplex RPC62/RPC39/RPC32. Two distinct isoforms of RPC32 are encoded in the human genome. RPC32α expression is highly regulated and found only in stem cells and transformed cells, whereas RPC32β is ubiquitously expressed in tissues. Here we identify a core-interacting domain of RPC32 sufficient for the interaction with RPC62. We present the crystal structure of a complex of RPC62 and the RPC32β core domain. RPC32β associates with the extended winged helix 1 and 2 and the coiled coil domain of RPC62 qualifying RPC32 as a molecular bridge in between RPC62 domains. The RPC62-RPC32 complex fit into EM data suggests a bi-functional role for RPC32 through interactions with the largest Pol III subunit and through solvent exposed residues. RPC32 positioning into Pol III suggests that subunit-specific contacts at the surface of the Pol III holoenzyme are critical for its function.
Source:Journal of Structural Biology
Author(s): Fanny Boissier, Hélène Dumay-Odelot, Martin Teichmann, Sébastien Fribourg
Transcription initiation by eukaryotic RNA polymerase (Pol) III relies on the subcomplex RPC62/RPC39/RPC32. Two distinct isoforms of RPC32 are encoded in the human genome. RPC32α expression is highly regulated and found only in stem cells and transformed cells, whereas RPC32β is ubiquitously expressed in tissues. Here we identify a core-interacting domain of RPC32 sufficient for the interaction with RPC62. We present the crystal structure of a complex of RPC62 and the RPC32β core domain. RPC32β associates with the extended winged helix 1 and 2 and the coiled coil domain of RPC62 qualifying RPC32 as a molecular bridge in between RPC62 domains. The RPC62-RPC32 complex fit into EM data suggests a bi-functional role for RPC32 through interactions with the largest Pol III subunit and through solvent exposed residues. RPC32 positioning into Pol III suggests that subunit-specific contacts at the surface of the Pol III holoenzyme are critical for its function.
Categories: Journal Articles