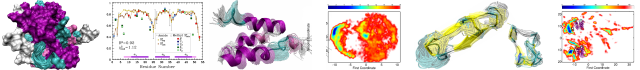Journal of Molecular Biology
DNA-Segment-Facilitated Dissociation of Fis and NHP6A from DNA Detected via Single-Molecule Mechanical Response
Publication date: 25 September 2015
Source:Journal of Molecular Biology, Volume 427, Issue 19
Author(s): Rebecca D. Giuntoli, Nora B. Linzer, Edward J. Banigan, Charles E. Sing, Monica Olvera de la Cruz, John S. Graham, Reid C. Johnson, John F. Marko
The rate of dissociation of a DNA–protein complex is often considered to be a property of that complex, without dependence on other nearby molecules in solution. We study the kinetics of dissociation of the abundant Escherichia coli nucleoid protein Fis from DNA, using a single-molecule mechanics assay. The rate of Fis dissociation from DNA is strongly dependent on the solution concentration of DNA. The off-rate (k off) of Fis from DNA shows an initially linear dependence on solution DNA concentration, characterized by an exchange rate of k ex ≈9×10−4 (ng/μl)−1 s−1 for 100mM univalent salt buffer, with a very small off-rate at zero DNA concentration. The off-rate saturates at approximately k off,max ≈8×10−3 s−1 for DNA concentrations above ≈20ng/μl. This exchange reaction depends mainly on DNA concentration with little dependence on the length of the DNA molecules in solution or on binding affinity, but this does increase with increasing salt concentration. We also show data for the yeast HMGB protein NHP6A showing a similar DNA-concentration-dependent dissociation effect, with faster rates suggesting generally weaker DNA binding by NHP6A relative to Fis. Our results are well described by a model with an intermediate partially dissociated state where the protein is susceptible to being captured by a second DNA segment, in the manner of “direct transfer” reactions studied for other DNA-binding proteins. This type of dissociation pathway may be important to protein–DNA binding kinetics in vivo where DNA concentrations are large.
Graphical abstract
Source:Journal of Molecular Biology, Volume 427, Issue 19
Author(s): Rebecca D. Giuntoli, Nora B. Linzer, Edward J. Banigan, Charles E. Sing, Monica Olvera de la Cruz, John S. Graham, Reid C. Johnson, John F. Marko
The rate of dissociation of a DNA–protein complex is often considered to be a property of that complex, without dependence on other nearby molecules in solution. We study the kinetics of dissociation of the abundant Escherichia coli nucleoid protein Fis from DNA, using a single-molecule mechanics assay. The rate of Fis dissociation from DNA is strongly dependent on the solution concentration of DNA. The off-rate (k off) of Fis from DNA shows an initially linear dependence on solution DNA concentration, characterized by an exchange rate of k ex ≈9×10−4 (ng/μl)−1 s−1 for 100mM univalent salt buffer, with a very small off-rate at zero DNA concentration. The off-rate saturates at approximately k off,max ≈8×10−3 s−1 for DNA concentrations above ≈20ng/μl. This exchange reaction depends mainly on DNA concentration with little dependence on the length of the DNA molecules in solution or on binding affinity, but this does increase with increasing salt concentration. We also show data for the yeast HMGB protein NHP6A showing a similar DNA-concentration-dependent dissociation effect, with faster rates suggesting generally weaker DNA binding by NHP6A relative to Fis. Our results are well described by a model with an intermediate partially dissociated state where the protein is susceptible to being captured by a second DNA segment, in the manner of “direct transfer” reactions studied for other DNA-binding proteins. This type of dissociation pathway may be important to protein–DNA binding kinetics in vivo where DNA concentrations are large.
Graphical abstract
Categories: Journal Articles
Coronin Enhances Actin Filament Severing by Recruiting Cofilin to Filament Sides and Altering F-Actin Conformation
Publication date: 25 September 2015
Source:Journal of Molecular Biology, Volume 427, Issue 19
Author(s): Mouna A. Mikati, Dennis Breitsprecher, Silvia Jansen, Emil Reisler, Bruce L. Goode
High rates of actin filament turnover are essential for many biological processes and require the activities of multiple actin-binding proteins working in concert. The mechanistic role of the actin filament severing protein cofilin is now firmly established; however, the contributions of other conserved disassembly-promoting factors including coronin have remained more obscure. Here, we have investigated the mechanism by which yeast coronin (Crn1) enhances F-actin turnover. Using multi-color total internal reflection fluorescence microscopy, we show that Crn1 enhances Cof1-mediated severing by accelerating Cof1 binding to actin filament sides. Further, using biochemical assays to interrogate F-actin conformation, we show that Crn1 alters longitudinal and lateral actin–actin contacts and restricts opening of the nucleotide-binding cleft in actin subunits. Moreover, Crn1 and Cof1 show opposite structural effects on F-actin yet synergize in promoting release of phalloidin from filaments, suggesting that Crn1/Cof1 co-decoration may increase local discontinuities in filament topology to enhance severing.
Graphical abstract
Source:Journal of Molecular Biology, Volume 427, Issue 19
Author(s): Mouna A. Mikati, Dennis Breitsprecher, Silvia Jansen, Emil Reisler, Bruce L. Goode
High rates of actin filament turnover are essential for many biological processes and require the activities of multiple actin-binding proteins working in concert. The mechanistic role of the actin filament severing protein cofilin is now firmly established; however, the contributions of other conserved disassembly-promoting factors including coronin have remained more obscure. Here, we have investigated the mechanism by which yeast coronin (Crn1) enhances F-actin turnover. Using multi-color total internal reflection fluorescence microscopy, we show that Crn1 enhances Cof1-mediated severing by accelerating Cof1 binding to actin filament sides. Further, using biochemical assays to interrogate F-actin conformation, we show that Crn1 alters longitudinal and lateral actin–actin contacts and restricts opening of the nucleotide-binding cleft in actin subunits. Moreover, Crn1 and Cof1 show opposite structural effects on F-actin yet synergize in promoting release of phalloidin from filaments, suggesting that Crn1/Cof1 co-decoration may increase local discontinuities in filament topology to enhance severing.
Graphical abstract
Categories: Journal Articles
Gradual Folding of an Off-Pathway Molten Globule Detected at the Single-Molecule Level
Publication date: 25 September 2015
Source:Journal of Molecular Biology, Volume 427, Issue 19
Author(s): Simon Lindhoud, Menahem Pirchi, Adrie H. Westphal, Gilad Haran, Carlo P.M. van Mierlo
Molten globules (MGs) are compact, partially folded intermediates that are transiently present during folding of many proteins. These intermediates reside on or off the folding pathway to native protein. Conformational evolution during folding of off-pathway MGs is largely unexplored. Here, we characterize the denaturant-dependent structure of apoflavodoxin's off-pathway MG. Using single-molecule fluorescence resonance energy transfer (smFRET), we follow conversion of unfolded species into MG down to denaturant concentrations that favor formation of native protein. Under strongly denaturing conditions, fluorescence resonance energy transfer histograms show a single peak, arising from unfolded protein. The smFRET efficiency distribution shifts to higher value upon decreasing denaturant concentration because the MG folds. Strikingly, upon approaching native conditions, the fluorescence resonance energy transfer efficiency of the MG rises above that of native protein. Thus, smFRET exposes the misfolded nature of apoflavodoxin's off-pathway MG. We show that conversion of unfolded into MG protein is a gradual, second-order-like process that simultaneously involves separate regions within the polypeptide.
Graphical abstract
Source:Journal of Molecular Biology, Volume 427, Issue 19
Author(s): Simon Lindhoud, Menahem Pirchi, Adrie H. Westphal, Gilad Haran, Carlo P.M. van Mierlo
Molten globules (MGs) are compact, partially folded intermediates that are transiently present during folding of many proteins. These intermediates reside on or off the folding pathway to native protein. Conformational evolution during folding of off-pathway MGs is largely unexplored. Here, we characterize the denaturant-dependent structure of apoflavodoxin's off-pathway MG. Using single-molecule fluorescence resonance energy transfer (smFRET), we follow conversion of unfolded species into MG down to denaturant concentrations that favor formation of native protein. Under strongly denaturing conditions, fluorescence resonance energy transfer histograms show a single peak, arising from unfolded protein. The smFRET efficiency distribution shifts to higher value upon decreasing denaturant concentration because the MG folds. Strikingly, upon approaching native conditions, the fluorescence resonance energy transfer efficiency of the MG rises above that of native protein. Thus, smFRET exposes the misfolded nature of apoflavodoxin's off-pathway MG. We show that conversion of unfolded into MG protein is a gradual, second-order-like process that simultaneously involves separate regions within the polypeptide.
Graphical abstract
Categories: Journal Articles
Highly Collapsed Conformation of the Initial Folding Intermediates of β-Lactoglobulin with Non-Native α-Helix
Publication date: 25 September 2015
Source:Journal of Molecular Biology, Volume 427, Issue 19
Author(s): Tsuyoshi Konuma, Kazumasa Sakurai, Masanori Yagi, Yuji Goto, Tetsuro Fujisawa, Satoshi Takahashi
In the folding of β-lactoglobulin (βLG), a predominantly β-sheet protein, a transient intermediate possessing an excess amount of non-native α-helix is formed within a few milliseconds. To characterize the early folding dynamics of βLG in terms of secondary structure content and compactness, we performed submillisecond-resolved circular dichroism (CD) and small-angle X-ray scattering (SAXS) measurements. Time-resolved CD after rapid dilution of urea showed non-native α-helix formation within 200μs. Time-resolved SAXS showed that the radius of gyration (R g) of the intermediate at 300μs was 23.3±0.7Å, indicating a considerable collapse from the unfolded state having R g of 35.1±7.1Å. Further compaction to R g of 21.2±0.3Å occurred with a time constant of 28±11ms. Pair distribution functions showed that the intermediate at 300μs comprises a single collapsed domain with a small fluctuating domain, which becomes more compact after the second collapse. Kinetic measurements in the presence of 2,2,2-trifluoroethanol showed that the intermediate at several milliseconds possessed an increased amount of α-helix but similar R g of 23.0±0.8Å, suggesting similarity of the shape of the intermediate in different solvents. Consequently, the initial collapse occurs globally to a compact state with a small fluctuating domain irrespective of the non-native α-helical contents. The second collapse of the fluctuating domain occurs in accordance with the reported stabilization of the non-native helix around strand A. The non-native helix around strand A might facilitate the formation of long-range contacts required for the folding of βLG.
Graphical abstract
Source:Journal of Molecular Biology, Volume 427, Issue 19
Author(s): Tsuyoshi Konuma, Kazumasa Sakurai, Masanori Yagi, Yuji Goto, Tetsuro Fujisawa, Satoshi Takahashi
In the folding of β-lactoglobulin (βLG), a predominantly β-sheet protein, a transient intermediate possessing an excess amount of non-native α-helix is formed within a few milliseconds. To characterize the early folding dynamics of βLG in terms of secondary structure content and compactness, we performed submillisecond-resolved circular dichroism (CD) and small-angle X-ray scattering (SAXS) measurements. Time-resolved CD after rapid dilution of urea showed non-native α-helix formation within 200μs. Time-resolved SAXS showed that the radius of gyration (R g) of the intermediate at 300μs was 23.3±0.7Å, indicating a considerable collapse from the unfolded state having R g of 35.1±7.1Å. Further compaction to R g of 21.2±0.3Å occurred with a time constant of 28±11ms. Pair distribution functions showed that the intermediate at 300μs comprises a single collapsed domain with a small fluctuating domain, which becomes more compact after the second collapse. Kinetic measurements in the presence of 2,2,2-trifluoroethanol showed that the intermediate at several milliseconds possessed an increased amount of α-helix but similar R g of 23.0±0.8Å, suggesting similarity of the shape of the intermediate in different solvents. Consequently, the initial collapse occurs globally to a compact state with a small fluctuating domain irrespective of the non-native α-helical contents. The second collapse of the fluctuating domain occurs in accordance with the reported stabilization of the non-native helix around strand A. The non-native helix around strand A might facilitate the formation of long-range contacts required for the folding of βLG.
Graphical abstract
Categories: Journal Articles
β-Structure within the Denatured State of the Helical Protein Domain BBL
Publication date: 25 September 2015
Source:Journal of Molecular Biology, Volume 427, Issue 19
Author(s): Lipi Thukral, Simone Schwarze, Isabella Daidone, Hannes Neuweiler
Protein denatured states are the origin of both healthy and toxic conformational species. Denatured states of ultrafast folding proteins are of interest in mechanistic studies because they are energetically close to the kinetic bottleneck of folding. However, their transient nature makes them elusive to experiment. Here, we generated the denatured state of the helical domain BBL that is poised to fold in microseconds by a single-point mutation and combined circular dichroism spectroscopy, single-molecule fluorescence fluctuation analysis, and computer simulation to characterize its structure and dynamics. Circular dichroism showed a largely unfolded ensemble with marginal helix but significant β-sheet content. Main-chain structure and dynamics were unaffected by side-chain interactions that stabilize the native state, as revealed by site-directed mutagenesis and nanosecond loop closure kinetics probed by fluorescence correlation spectroscopy. Replica-exchange and constant-temperature molecular dynamics simulations showed a highly collapsed, hydrogen-bonded denatured state containing turn and β-sheet structure and few nucleating helices in an otherwise unfolded ensemble. An irregular β-hairpin element that connects helices in the native fold was poised to be formed. The surprising observation of β-structure in regions that form helices in the native state is reconciled by a generic low-energy pathway from the northwest quadrant of Ramachandran space to the helical basin present under folding conditions, proposed recently. Our results show that, indeed, rapid nucleation of helix emanates from β-structure formed early within a collapsed ensemble of unfolded conformers.
Graphical abstract
Source:Journal of Molecular Biology, Volume 427, Issue 19
Author(s): Lipi Thukral, Simone Schwarze, Isabella Daidone, Hannes Neuweiler
Protein denatured states are the origin of both healthy and toxic conformational species. Denatured states of ultrafast folding proteins are of interest in mechanistic studies because they are energetically close to the kinetic bottleneck of folding. However, their transient nature makes them elusive to experiment. Here, we generated the denatured state of the helical domain BBL that is poised to fold in microseconds by a single-point mutation and combined circular dichroism spectroscopy, single-molecule fluorescence fluctuation analysis, and computer simulation to characterize its structure and dynamics. Circular dichroism showed a largely unfolded ensemble with marginal helix but significant β-sheet content. Main-chain structure and dynamics were unaffected by side-chain interactions that stabilize the native state, as revealed by site-directed mutagenesis and nanosecond loop closure kinetics probed by fluorescence correlation spectroscopy. Replica-exchange and constant-temperature molecular dynamics simulations showed a highly collapsed, hydrogen-bonded denatured state containing turn and β-sheet structure and few nucleating helices in an otherwise unfolded ensemble. An irregular β-hairpin element that connects helices in the native fold was poised to be formed. The surprising observation of β-structure in regions that form helices in the native state is reconciled by a generic low-energy pathway from the northwest quadrant of Ramachandran space to the helical basin present under folding conditions, proposed recently. Our results show that, indeed, rapid nucleation of helix emanates from β-structure formed early within a collapsed ensemble of unfolded conformers.
Graphical abstract
Categories: Journal Articles
α-Lactalbumin:Oleic Acid Complex Spontaneously Delivers Oleic Acid to Artificial and Erythrocyte Membranes
Publication date: 25 September 2015
Source:Journal of Molecular Biology, Volume 427, Issue 19
Author(s): Hanzhen Wen, Øyvind Strømland, Øyvind Halskau
Human α-lactalbumin made lethal to tumor cells (HAMLET) is a tumoricidal complex consisting of human α-lactalbumin and multiple oleic acids (OAs). OA has been shown to play a key role in the activity of HAMLET and its related complexes, generally known as protein–fatty acid (PFA) complexes. In contrast to what is known about the fate of the protein component of such complexes, information about what happens to OA during their action is still lacking. We monitored the membrane, OA and protein components of bovine α-lactalbumin complexed with OA (BLAOA; a HAMLET-like substance) and how they associate with each other. Using ultracentrifugation, we found that the OA and lipid components follow each other closely. We then firmly identify a transfer of OA from BLAOA to both artificial and erythrocyte membranes, indicating that natural cells respond similarly to BLAOA treatment as artificial membranes. Uncomplexed OA is unable to similarly affect membranes at the conditions tested, even at elevated concentrations. Thus, BLAOA can spontaneously transfer OA to a lipid membrane. After the interaction with the membrane, the protein is likely to have lost most or all of its OA. We suggest a mechanism for passive import of mainly uncomplexed protein into cells, using existing models for OA's effect on membranes. Our results are consistent with a membrane destabilization mediated predominantly by OA insertion being a significant contribution to PFA cytotoxicity.
Graphical abstract
Source:Journal of Molecular Biology, Volume 427, Issue 19
Author(s): Hanzhen Wen, Øyvind Strømland, Øyvind Halskau
Human α-lactalbumin made lethal to tumor cells (HAMLET) is a tumoricidal complex consisting of human α-lactalbumin and multiple oleic acids (OAs). OA has been shown to play a key role in the activity of HAMLET and its related complexes, generally known as protein–fatty acid (PFA) complexes. In contrast to what is known about the fate of the protein component of such complexes, information about what happens to OA during their action is still lacking. We monitored the membrane, OA and protein components of bovine α-lactalbumin complexed with OA (BLAOA; a HAMLET-like substance) and how they associate with each other. Using ultracentrifugation, we found that the OA and lipid components follow each other closely. We then firmly identify a transfer of OA from BLAOA to both artificial and erythrocyte membranes, indicating that natural cells respond similarly to BLAOA treatment as artificial membranes. Uncomplexed OA is unable to similarly affect membranes at the conditions tested, even at elevated concentrations. Thus, BLAOA can spontaneously transfer OA to a lipid membrane. After the interaction with the membrane, the protein is likely to have lost most or all of its OA. We suggest a mechanism for passive import of mainly uncomplexed protein into cells, using existing models for OA's effect on membranes. Our results are consistent with a membrane destabilization mediated predominantly by OA insertion being a significant contribution to PFA cytotoxicity.
Graphical abstract
Categories: Journal Articles
Encapsulation as a strategy for the design of biological compartmentalization
Publication date: Available online 25 September 2015
Source:Journal of Molecular Biology
Author(s): Tobias W. Giessen, Pamela A. Silver
Compartmentalization is one of the defining features of life. Through intracellular spatial control, cells are able to organize and regulate their metabolism. One of the most broadly used organizational principles in nature is encapsulation. Cellular processes can either be encapsulated within membrane-bound organelles or proteinaceous compartments that create distinct microenvironments optimized for a given task. Further challenges addressed through intracellular compartmentalization are toxic or volatile pathway intermediates, slow turnover rates, and competing side reactions. This review highlights a selection of naturally occurring membrane- and protein-based encapsulation systems in microbes and their recent applications and emerging opportunities in synthetic biology. We focus on examples that use engineered cellular organization to control metabolic pathway flux for the production of useful compounds and materials.
Graphical abstract
Source:Journal of Molecular Biology
Author(s): Tobias W. Giessen, Pamela A. Silver
Compartmentalization is one of the defining features of life. Through intracellular spatial control, cells are able to organize and regulate their metabolism. One of the most broadly used organizational principles in nature is encapsulation. Cellular processes can either be encapsulated within membrane-bound organelles or proteinaceous compartments that create distinct microenvironments optimized for a given task. Further challenges addressed through intracellular compartmentalization are toxic or volatile pathway intermediates, slow turnover rates, and competing side reactions. This review highlights a selection of naturally occurring membrane- and protein-based encapsulation systems in microbes and their recent applications and emerging opportunities in synthetic biology. We focus on examples that use engineered cellular organization to control metabolic pathway flux for the production of useful compounds and materials.
Graphical abstract
Categories: Journal Articles
The antibody light chain linker is important for domain stability and amyloid formation
Publication date: Available online 25 September 2015
Source:Journal of Molecular Biology
Author(s): Cardine N. Nokwe, Manuel Hora, Martin Zacharias, Hisashi Yagi, Christine John, Bernd Reif, Yuji Goto, Johannes Buchner
The association of light chains (LCs) and heavy chains is the basis for functional antibodies that are essential for adaptive immune responses. However, in some cases, LCs and especially fragments consisting of the LC variable (VL) domain are pathologically deposited in fatal aggregation diseases. The two domains of the LC are connected by a highly conserved linker. We show here that, unexpectedly, the linker residue Arg108 affects the conformational stability and folding of both VLκ and LC constant (CLκ) domains. Interestingly, the extension of VL by Arg108 results in its resistance to amyloid formation, which suggests that the nature of the truncation of the LC plays a crucial role in disease progression. Increased solvation due to the exposed charged C-terminal Arg108 residue explains its stabilizing effects on the VL domain. For the CL domain, the interaction of N-terminal loop residues with Arg108 is important for the integrity of the domain, as the disruption of this interaction results in fluctuation, partial opening of the protein’s interior and the exposure of hydrophobic residues that destabilize the domain. This establishes new principles for antibody domain architecture and amyloidogenicity.
Graphical abstract
Source:Journal of Molecular Biology
Author(s): Cardine N. Nokwe, Manuel Hora, Martin Zacharias, Hisashi Yagi, Christine John, Bernd Reif, Yuji Goto, Johannes Buchner
The association of light chains (LCs) and heavy chains is the basis for functional antibodies that are essential for adaptive immune responses. However, in some cases, LCs and especially fragments consisting of the LC variable (VL) domain are pathologically deposited in fatal aggregation diseases. The two domains of the LC are connected by a highly conserved linker. We show here that, unexpectedly, the linker residue Arg108 affects the conformational stability and folding of both VLκ and LC constant (CLκ) domains. Interestingly, the extension of VL by Arg108 results in its resistance to amyloid formation, which suggests that the nature of the truncation of the LC plays a crucial role in disease progression. Increased solvation due to the exposed charged C-terminal Arg108 residue explains its stabilizing effects on the VL domain. For the CL domain, the interaction of N-terminal loop residues with Arg108 is important for the integrity of the domain, as the disruption of this interaction results in fluctuation, partial opening of the protein’s interior and the exposure of hydrophobic residues that destabilize the domain. This establishes new principles for antibody domain architecture and amyloidogenicity.
Graphical abstract
Categories: Journal Articles
AlloRep: a repository of sequence, structural and mutagenesis data for the LacI/GalR transcription regulators
Publication date: Available online 25 September 2015
Source:Journal of Molecular Biology
Author(s): Filipa L. Sousa, Daniel J. Parente, David L. Shis, Jacob A. Hessman, Allen Chazelle, Matthew R. Bennett, Sarah A. Teichmann, Liskin Swint-Kruse
Protein families evolve functional variation by accumulating point-mutations at functionally-important amino acid positions. Homologs in the LacI/GalR family of transcription regulators have evolved to bind diverse DNA sequences and allosteric regulatory molecules. In addition to playing key roles in bacterial metabolism, these proteins have been widely used as a model family for benchmarking structural and functional prediction algorithms. We have collected manually-curated sequence alignments for >3000 sequences, in vivo phenotypic and biochemical data for >5750 LacI/GalR mutational variants, and non-covalent residue contact networks for 65 LacI/GalR homolog structures. Using this rich data resource, we compared the non-covalent residue contact networks of the LacI/GalR subfamilies to design and experimentally validate an allosteric mutant of a synthetic LacI/GalR repressor for use in biotechnology. The AlloRep database (freely available at www.AlloRep.org) is a key resource for future evolutionary studies of LacI/GalR homologs and for benchmarking computational predictions of functional change.
Graphical abstract
Source:Journal of Molecular Biology
Author(s): Filipa L. Sousa, Daniel J. Parente, David L. Shis, Jacob A. Hessman, Allen Chazelle, Matthew R. Bennett, Sarah A. Teichmann, Liskin Swint-Kruse
Protein families evolve functional variation by accumulating point-mutations at functionally-important amino acid positions. Homologs in the LacI/GalR family of transcription regulators have evolved to bind diverse DNA sequences and allosteric regulatory molecules. In addition to playing key roles in bacterial metabolism, these proteins have been widely used as a model family for benchmarking structural and functional prediction algorithms. We have collected manually-curated sequence alignments for >3000 sequences, in vivo phenotypic and biochemical data for >5750 LacI/GalR mutational variants, and non-covalent residue contact networks for 65 LacI/GalR homolog structures. Using this rich data resource, we compared the non-covalent residue contact networks of the LacI/GalR subfamilies to design and experimentally validate an allosteric mutant of a synthetic LacI/GalR repressor for use in biotechnology. The AlloRep database (freely available at www.AlloRep.org) is a key resource for future evolutionary studies of LacI/GalR homologs and for benchmarking computational predictions of functional change.
Graphical abstract
Categories: Journal Articles
UbSRD: The Ubiquitin Structural Relational Database
Publication date: Available online 25 September 2015
Source:Journal of Molecular Biology
Author(s): Joseph S. Harrison, Tim M. Jacobs, Kevin Houlihan, Koenraad Van Doorslaer, Brian Kuhlman
The structurally defined ubiquitin-like homology fold (UBL) can engage in several unique protein-protein interactions (PPI) and many of these complexes have been characterized with high-resolution techniques. Using Rosetta's structural classification tools we have created the Ubiquitin Structural Relational Database (UbSRD), an SQL database of features for all 509 UBL-containing structures in the PDB, allowing users to browse these structures by PPI and providing a platform for quantitative analysis of structural features. We used UbSRD to define the recognition features of ubiquitin (UBQ) and SUMO observed in the PDB and the orientation of the UBQ tail while interacting with certain types of proteins. While some of the interaction surfaces on UBQ and SUMO overlap, each molecule has distinct features that aid in molecular discrimination. Additionally, we find that the UBQ tail is malleable and can adopt a variety of conformations upon binding. UbSRD is accessible as an online resource at rosettadesign.med.unc.edu/ubsrd.
Source:Journal of Molecular Biology
Author(s): Joseph S. Harrison, Tim M. Jacobs, Kevin Houlihan, Koenraad Van Doorslaer, Brian Kuhlman
The structurally defined ubiquitin-like homology fold (UBL) can engage in several unique protein-protein interactions (PPI) and many of these complexes have been characterized with high-resolution techniques. Using Rosetta's structural classification tools we have created the Ubiquitin Structural Relational Database (UbSRD), an SQL database of features for all 509 UBL-containing structures in the PDB, allowing users to browse these structures by PPI and providing a platform for quantitative analysis of structural features. We used UbSRD to define the recognition features of ubiquitin (UBQ) and SUMO observed in the PDB and the orientation of the UBQ tail while interacting with certain types of proteins. While some of the interaction surfaces on UBQ and SUMO overlap, each molecule has distinct features that aid in molecular discrimination. Additionally, we find that the UBQ tail is malleable and can adopt a variety of conformations upon binding. UbSRD is accessible as an online resource at rosettadesign.med.unc.edu/ubsrd.
Categories: Journal Articles
Contact-dependent growth inhibition (CDI) and CdiB/CdiA two-partner secretion proteins
Publication date: Available online 24 September 2015
Source:Journal of Molecular Biology
Author(s): Julia L.E. Willett, Zachary C. Ruhe, Celia W. Goulding, David A. Low, Christopher S. Hayes
Bacteria have developed several strategies to communicate and compete with one another in complex environments. One important mechanism of inter-bacterial competition is contact-dependent growth inhibition (CDI), in which some Gram-negative bacteria use CdiB/CdiA two-partner secretion proteins to suppress the growth of neighboring target cells. CdiB is an Omp85 outer-membrane protein that exports and assembles CdiA exoproteins onto the inhibitor-cell surface. CdiA binds to receptors on susceptible bacteria and subsequently delivers its C-terminal toxin domain (CdiA-CT) into the target cell. CDI systems also encode CdiI immunity proteins, which specifically bind to the CdiA-CT and neutralize its toxin activity, thereby protecting CDI+ cells from auto-inhibition. Remarkably, CdiA-CT sequences are highly variable between bacteria, as are the corresponding CdiI immunity proteins. Variations in CDI toxin/immunity proteins suggest that these systems function in bacterial self/nonself recognition and thereby play an important role in microbial communities. In this review, we discuss recent advances in the biochemistry, structural biology and physiology of CDI.
Graphical abstract
Source:Journal of Molecular Biology
Author(s): Julia L.E. Willett, Zachary C. Ruhe, Celia W. Goulding, David A. Low, Christopher S. Hayes
Bacteria have developed several strategies to communicate and compete with one another in complex environments. One important mechanism of inter-bacterial competition is contact-dependent growth inhibition (CDI), in which some Gram-negative bacteria use CdiB/CdiA two-partner secretion proteins to suppress the growth of neighboring target cells. CdiB is an Omp85 outer-membrane protein that exports and assembles CdiA exoproteins onto the inhibitor-cell surface. CdiA binds to receptors on susceptible bacteria and subsequently delivers its C-terminal toxin domain (CdiA-CT) into the target cell. CDI systems also encode CdiI immunity proteins, which specifically bind to the CdiA-CT and neutralize its toxin activity, thereby protecting CDI+ cells from auto-inhibition. Remarkably, CdiA-CT sequences are highly variable between bacteria, as are the corresponding CdiI immunity proteins. Variations in CDI toxin/immunity proteins suggest that these systems function in bacterial self/nonself recognition and thereby play an important role in microbial communities. In this review, we discuss recent advances in the biochemistry, structural biology and physiology of CDI.
Graphical abstract
Categories: Journal Articles
Navigating towards an understanding of the role of Regulator of Calcineurin in thermotaxis
Publication date: Available online 24 September 2015
Source:Journal of Molecular Biology
Author(s): Tami Kingsbury
Source:Journal of Molecular Biology
Author(s): Tami Kingsbury
Categories: Journal Articles
Protein-protein interfaces in viral capsids are structurally unique
Publication date: Available online 12 September 2015
Source:Journal of Molecular Biology
Author(s): Shanshan Cheng, Charles L. Brooks
Viral capsids exhibit elaborate and symmetrical architectures of defined sizes and remarkable mechanical properties not seen with cellular macromolecular complexes. Given the uniqueness of the higher order organization of viral capsid proteins in the virosphere, we explored the question of whether the patterns of protein-protein interactions within viral capsids are distinct from those in generic protein complexes. Our comparative analysis involving a non-redundant set of 551 inter-subunit interfaces in viral capsids from VIPERdb and 20014 protein-protein interfaces in non-capsid protein complexes from the PDB found 418 generic protein-protein interfaces that share similar physicochemical patterns with some protein-protein interfaces in the capsid set, using the program PCalign we developed for comparing protein-protein interfaces. This overlap in the structural space of protein-protein interfaces is significantly small, with a p-value < 0.0001, based on a permutation test on the total set of protein-protein interfaces. Furthermore, the generic protein-protein interfaces that bear similarity in their spatial and chemical arrangement with capsid ones are mostly small in size with fewer than 20 interfacial residues, which results from the relatively limited choices of natural design for small interfaces, rather than having significant biological implications in terms of functional relationships. We conclude based on this study that protein-protein interfaces in viral capsids are non-representative of patterns in the smaller, more compact cellular protein complexes. Our finding highlights the design principle of building large biological containers from repeated, self-assembling units, and provides insights into specific targets for antiviral drug design for improved efficacy.
Graphical abstract
Source:Journal of Molecular Biology
Author(s): Shanshan Cheng, Charles L. Brooks
Viral capsids exhibit elaborate and symmetrical architectures of defined sizes and remarkable mechanical properties not seen with cellular macromolecular complexes. Given the uniqueness of the higher order organization of viral capsid proteins in the virosphere, we explored the question of whether the patterns of protein-protein interactions within viral capsids are distinct from those in generic protein complexes. Our comparative analysis involving a non-redundant set of 551 inter-subunit interfaces in viral capsids from VIPERdb and 20014 protein-protein interfaces in non-capsid protein complexes from the PDB found 418 generic protein-protein interfaces that share similar physicochemical patterns with some protein-protein interfaces in the capsid set, using the program PCalign we developed for comparing protein-protein interfaces. This overlap in the structural space of protein-protein interfaces is significantly small, with a p-value < 0.0001, based on a permutation test on the total set of protein-protein interfaces. Furthermore, the generic protein-protein interfaces that bear similarity in their spatial and chemical arrangement with capsid ones are mostly small in size with fewer than 20 interfacial residues, which results from the relatively limited choices of natural design for small interfaces, rather than having significant biological implications in terms of functional relationships. We conclude based on this study that protein-protein interfaces in viral capsids are non-representative of patterns in the smaller, more compact cellular protein complexes. Our finding highlights the design principle of building large biological containers from repeated, self-assembling units, and provides insights into specific targets for antiviral drug design for improved efficacy.
Graphical abstract
Categories: Journal Articles
Editorial Board
Publication date: 11 September 2015
Source:Journal of Molecular Biology, Volume 427, Issue 18
Source:Journal of Molecular Biology, Volume 427, Issue 18
Categories: Journal Articles
Contents List
Publication date: 11 September 2015
Source:Journal of Molecular Biology, Volume 427, Issue 18
Source:Journal of Molecular Biology, Volume 427, Issue 18
Categories: Journal Articles
The network of molecular chaperones: insights in the cellular proteostasis machinery
Publication date: 11 September 2015
Source:Journal of Molecular Biology, Volume 427, Issue 18
Author(s): Marina Ostankovitch, Johannes Buchner
Source:Journal of Molecular Biology, Volume 427, Issue 18
Author(s): Marina Ostankovitch, Johannes Buchner
Categories: Journal Articles
Mechanistic Asymmetry in Hsp90 Dimers
Publication date: 11 September 2015
Source:Journal of Molecular Biology, Volume 427, Issue 18
Author(s): Julia M. Flynn, Parul Mishra, Daniel N.A. Bolon
Hsp90 is a molecular chaperone that facilitates the maturation of signaling proteins including many kinases and steroid hormone receptors. Through these client proteins, Hsp90 is a key mediator of many physiological processes and has emerged as a promising drug target in cancer. Additionally, Hsp90 can mask or potentiate the impact of mutations in clients with remarkable influence on evolutionary adaptations. The influential roles of Hsp90 in biology and disease have stimulated extensive research into the molecular mechanism of this chaperone. These studies have shown that Hsp90 is a homodimeric protein that requires ATP hydrolysis and a host of accessory proteins termed co-chaperones to facilitate the maturation of clients to their active states. Flexible hinge regions between its three structured domains enable Hsp90 to sample dramatically distinct conformations that are influenced by nucleotide, client, and co-chaperone binding. While it is clear that Hsp90 can exist in symmetrical conformations, recent studies have indicated that this homodimeric chaperone can also assume a variety of asymmetric conformations and complexes that are important for client maturation. The visualization of Hsp90-client complexes at high resolution together with tools to independently manipulate each subunit in the Hsp90 dimer are providing new insights into the asymmetric function of each subunit during client maturation.
Graphical abstract
Source:Journal of Molecular Biology, Volume 427, Issue 18
Author(s): Julia M. Flynn, Parul Mishra, Daniel N.A. Bolon
Hsp90 is a molecular chaperone that facilitates the maturation of signaling proteins including many kinases and steroid hormone receptors. Through these client proteins, Hsp90 is a key mediator of many physiological processes and has emerged as a promising drug target in cancer. Additionally, Hsp90 can mask or potentiate the impact of mutations in clients with remarkable influence on evolutionary adaptations. The influential roles of Hsp90 in biology and disease have stimulated extensive research into the molecular mechanism of this chaperone. These studies have shown that Hsp90 is a homodimeric protein that requires ATP hydrolysis and a host of accessory proteins termed co-chaperones to facilitate the maturation of clients to their active states. Flexible hinge regions between its three structured domains enable Hsp90 to sample dramatically distinct conformations that are influenced by nucleotide, client, and co-chaperone binding. While it is clear that Hsp90 can exist in symmetrical conformations, recent studies have indicated that this homodimeric chaperone can also assume a variety of asymmetric conformations and complexes that are important for client maturation. The visualization of Hsp90-client complexes at high resolution together with tools to independently manipulate each subunit in the Hsp90 dimer are providing new insights into the asymmetric function of each subunit during client maturation.
Graphical abstract
Categories: Journal Articles
Reaction Cycle of Chaperonin GroEL via Symmetric “Football” Intermediate
Publication date: 11 September 2015
Source:Journal of Molecular Biology, Volume 427, Issue 18
Author(s): Hideki Taguchi
Chaperonin GroEL is an essential chaperone that assists in protein folding in the cell. Since one GroEL ring binds one GroES heptamer, the GroEL double ring permits the formation of two types of GroEL:GroES complexes: asymmetric 1:1 “bullet”-shaped and symmetric 1:2 “football”-shaped GroEL:GroES2 complexes. There have been continuing debates about the mechanism and which complex is critical to the chaperonin-assisted folding. In this review, I summarize the recent progress on the football complex.
Graphical abstract
Source:Journal of Molecular Biology, Volume 427, Issue 18
Author(s): Hideki Taguchi
Chaperonin GroEL is an essential chaperone that assists in protein folding in the cell. Since one GroEL ring binds one GroES heptamer, the GroEL double ring permits the formation of two types of GroEL:GroES complexes: asymmetric 1:1 “bullet”-shaped and symmetric 1:2 “football”-shaped GroEL:GroES2 complexes. There have been continuing debates about the mechanism and which complex is critical to the chaperonin-assisted folding. In this review, I summarize the recent progress on the football complex.
Graphical abstract
Categories: Journal Articles
The Mechanism and Function of Group II Chaperonins
Publication date: 11 September 2015
Source:Journal of Molecular Biology, Volume 427, Issue 18
Author(s): Tom Lopez, Kevin Dalton, Judith Frydman
Protein folding in the cell requires the assistance of enzymes collectively called chaperones. Among these, the chaperonins are 1-MDa ring-shaped oligomeric complexes that bind unfolded polypeptides and promote their folding within an isolated chamber in an ATP-dependent manner. Group II chaperonins, found in archaea and eukaryotes, contain a built-in lid that opens and closes over the central chamber. In eukaryotes, the chaperonin TRiC/CCT is hetero-oligomeric, consisting of two stacked rings of eight paralogous subunits each. TRiC facilitates folding of approximately 10% of the eukaryotic proteome, including many cytoskeletal components and cell cycle regulators. Folding of many cellular substrates of TRiC cannot be assisted by any other chaperone. A complete structural and mechanistic understanding of this highly conserved and essential chaperonin remains elusive. However, recent work is beginning to shed light on key aspects of chaperonin function and how their unique properties underlie their contribution to maintaining cellular proteostasis.
Graphical abstract
Source:Journal of Molecular Biology, Volume 427, Issue 18
Author(s): Tom Lopez, Kevin Dalton, Judith Frydman
Protein folding in the cell requires the assistance of enzymes collectively called chaperones. Among these, the chaperonins are 1-MDa ring-shaped oligomeric complexes that bind unfolded polypeptides and promote their folding within an isolated chamber in an ATP-dependent manner. Group II chaperonins, found in archaea and eukaryotes, contain a built-in lid that opens and closes over the central chamber. In eukaryotes, the chaperonin TRiC/CCT is hetero-oligomeric, consisting of two stacked rings of eight paralogous subunits each. TRiC facilitates folding of approximately 10% of the eukaryotic proteome, including many cytoskeletal components and cell cycle regulators. Folding of many cellular substrates of TRiC cannot be assisted by any other chaperone. A complete structural and mechanistic understanding of this highly conserved and essential chaperonin remains elusive. However, recent work is beginning to shed light on key aspects of chaperonin function and how their unique properties underlie their contribution to maintaining cellular proteostasis.
Graphical abstract
Categories: Journal Articles
The Chemical Biology of Molecular Chaperones—Implications for Modulation of Proteostasis
Publication date: 11 September 2015
Source:Journal of Molecular Biology, Volume 427, Issue 18
Author(s): Kristoffer R. Brandvold, Richard I. Morimoto
Protein homeostasis (proteostasis) is inextricably tied to cellular health and organismal lifespan. Aging, exposure to physiological and environmental stress, and expression of mutant and metastable proteins can cause an imbalance in the protein-folding landscape, which results in the formation of non-native protein aggregates that challenge the capacity of the proteostasis network (PN), increasing the risk for diseases associated with misfolding, aggregation, and aberrant regulation of cell stress responses. Molecular chaperones have central roles in each of the arms of the PN (protein synthesis, folding, disaggregation, and degradation), leading to the proposal that modulation of chaperone function could have therapeutic benefits for the large and growing family of diseases of protein conformation including neurodegeneration, metabolic diseases, and cancer. In this review, we will discuss the current strategies used to tune the PN through targeting molecular chaperones and assess the potential of the chemical biology of proteostasis.
Graphical abstract
Source:Journal of Molecular Biology, Volume 427, Issue 18
Author(s): Kristoffer R. Brandvold, Richard I. Morimoto
Protein homeostasis (proteostasis) is inextricably tied to cellular health and organismal lifespan. Aging, exposure to physiological and environmental stress, and expression of mutant and metastable proteins can cause an imbalance in the protein-folding landscape, which results in the formation of non-native protein aggregates that challenge the capacity of the proteostasis network (PN), increasing the risk for diseases associated with misfolding, aggregation, and aberrant regulation of cell stress responses. Molecular chaperones have central roles in each of the arms of the PN (protein synthesis, folding, disaggregation, and degradation), leading to the proposal that modulation of chaperone function could have therapeutic benefits for the large and growing family of diseases of protein conformation including neurodegeneration, metabolic diseases, and cancer. In this review, we will discuss the current strategies used to tune the PN through targeting molecular chaperones and assess the potential of the chemical biology of proteostasis.
Graphical abstract
Categories: Journal Articles